





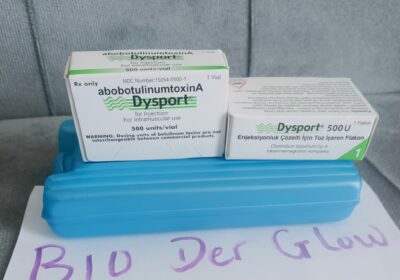
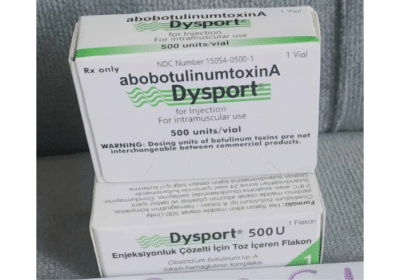
Dysport 500 Units Powder For Solution For Injection | Bioderglow
Website: bioderglow.com
Tell: +13053961530
Telegram: @bioderglow
Dysport has been approved by US FDA in 2009 for carrying out the treatment of cervical dystonia or blepharospasm in the adults, apart from cosmetic treatment.
These have been running successfully over the years.
Each sterile vial contains 500 units of lyophilized abobotulinumtoxinA.
Nonmedicinal ingredients: human serum albumin and lactose.
Botulinum toxin belongs to the class of medications called neuromuscular paralytic agents.
It blocks the nerves that are responsible for muscle activity.
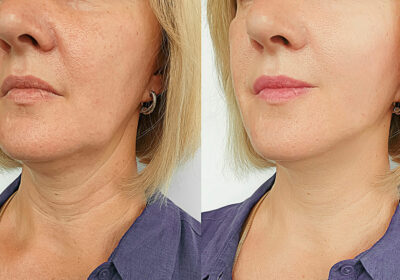
For cosmetic purposes, it can be used to smooth out facial lines and wrinkles, such as those that form between the eyebrows, on the forehead, and around the eyes (crow’s feet).
It gives skin a smoother appearance by relaxing the muscles in the area where it was injected.
It may also be used to treat cervical dystonia, also known as spasmodic torticollis, a condition in which the muscles of the neck stay in a state of contraction, and to treat focal spasticity such as arm spasms after a stroke. Dysport 500 unit Powder.
Possible side effects.
Drooping of the upper eyelid
Face pain
General feeling of being unwell
Headache
Muscle weakness at the injection site
Nausea
Pain, tenderness or bruising, burning, swelling, or stinging at the injection site
Redness of the skin
Stuffy nose
Tightness of the skin
How to use Dysport 500 unit
Dosage Forms & Strengths
Injection, powder for reconstitution
300 units/vial
500 units/vial
Cervical Dystonia
Indicated for cervical dystonia
Initial: 500 unit IM divided among affected muscles
Retreat every 12-16 weeks or longer: 250-1000 unit IM
Titrate in 250-unit steps
Spasticity
Indicated for treatment of spasticity in adults
Select dose based on muscles affected, severity of muscle activity, prior response to treatment, and adverse event history (EMG guidance recommended)
Do not inject >1 mL at any single injection site; the maximum total dose (upper and lower limb combined) is 1500 units
Repeat treatment when the effect of a previous injection has diminished, but no sooner than 12 weeks after the previous injection
A majority of patients are retreated between 12-16 weeks, although some may have a longer response (eg, 20 wk)
Upper limb spasticity
Dose per muscle
In the clinical trial, doses of 500-1000 units were divided among selected upper limb muscles at a given treatment session
Flexor carpi radialis: 100-200 units in 1-2 sites
Flexor carpi ulnaris: 100-200 units in 1-2 sites
Flexor digitorum profundus: 100-200 units in 1-2 sites
Flexor digitorum sublimis: 100-200 units in 1-2 sites
Brachialis: 200-400 units in 1-2 sites
Brachioradialis: 100-200 units in 1-2 sites
Biceps brachii: 200-400 units divided in 1-2 sites
Pronator Teres: 100-200 units in 1 site
Lower limb spasticity
Dose per muscle
In the clinical trial, doses of 1000-1500 units were divided among selected lower limb muscles at a given treatment session
Gastrocnemius medial head: 100-150 units in 1 site
Gastrocnemius lateral head: 100-150 units in 1 site
Soleus: 330-500 units in 3 sites
Tibialis posterior: 200-300 units in 2 sites
Flexor digitorum longus: 130-200 units in 1-2 sites
Flexor hallucis longus: 70-200 units in 1 site
Glabellar Lines
Indicated for temporary improvement in the appearance of moderate to severe glabellar lines associated with procerus and corrugator muscle activity in adults aged <65 years
50 units total divided in 5 equal doses IM to affected muscles
Retreat no sooner than 3 months
Dosage Modifications
Renal or hepatic impairment: No dose adjustment necessary
Essential Blepharospasm (Orphan)
Orphan indication sponsor
Porton International, Inc; 1155 15th Street, N.W., #315; Washington, DC 20005
Spasmodic Torticollis (Orphan)
Treatment of spasmodic torticollis (cervical dystonia)
Orphan indication sponsor
Ipsen Biopharm Limited; 1 Bath Road Maidenhead, Berkshire, SL6 4UH; UK
Website – https://bioderglow.com/

Seller Information
Please login to view the seller information.
Business Hours
| Open | Close | ||
|---|---|---|---|
| Monday | Open (24 Hours) | ||
| Tuesday | Open (24 Hours) | ||
| Wednesday | Open (24 Hours) | ||
| Thursday | Open Today (24 Hours) | ||
| Friday | Open (24 Hours) | ||
| Saturday | Open (24 Hours) | ||
| Sunday | Open (24 Hours) | ||
Location
Related Ads
Relieve Digital Eye Strain with ITone Herbal Eye Drops
- 1 week ago
- Kolkata - Baghbazar, West Bengal, India
- 15 Views
Relieve Digital Eye Strain with I-Tone Herbal Eye Drops
- 2 weeks ago
- Kolkata - Ballygunge, West Bengal, India
- 21 Views
L-Arginine Proanthocyanidins Sachets | Arnum
- 3 weeks ago
- Panchkula City, Haryana, India
- 16 Views
ACECLOFENAC PARACETAMOL TABLETS | ARA-P TABLETS
- 3 weeks ago
- Panchkula City, Haryana, India
- 17 Views
Flavoxate Hydrochloride Tablets | Cnof F 200 Tablets
- 3 weeks ago
- Panchkula City, Haryana, India
- 14 Views
Tamsulosin Hydrochloride and Dutasteride Tablets | Peflow
- 3 weeks ago
- Panchkula City, Haryana, India
- 11 Views
ACECLOFENAC DROTAVERINE HYDROCHLORIDE TABLETS | ATEKAR-SPAS TABLET
- 4 weeks ago
- Panchkula City, Haryana, India
- 15 Views
ACECLOFENAC PARACETAMOL CHLORZOXAZONE TABLETS | ARA-MR
- 4 weeks ago
- Panchkula City, Haryana, India
- 16 Views
Acebrophylline and Acetylcysteine Tablets | Brofil-N
- 1 month ago
- Panchkula City, Haryana, India
- 14 Views
Polymyxin B 500000 Units Injection | Plenum Biotech
- 1 month ago
- Panchkula City, Haryana, India
- 22 Views
Best Supplier of SoviHep V in India | Gandhi Medicos
- 1 month ago
- Jhandewalan, Delhi, India
- 17 Views
Intelicure Lifesciences – Leading The Way in Critical Care Medicine
- 1 month ago
- Chandigarh City, Chandigarh, India
- 15 Views
Tucatinib 150mg Up To 15% Off | Magicine Pharma
- 1 month ago
- Delhi Cantonment, Delhi, India
- 22 Views
Best Medicine For Sleep by The Uplife
- 2 months ago
- Panaji (Panjim), Goa, India
- 28 Views
Shingles Vaccine in Hyderabad
- 2 months ago
- Hyderabad - Punjagutta, Telangana, India
- 24 Views
IV Antibiotics at Home in Hyderabad
- 2 months ago
- Hyderabad - Ameerpet, Telangana, India
- 19 Views
Best CEFPORON 100 DT Tablets | Aeron Remedies
- 2 months ago
- Amb, Himachal Pradesh, India
- 50 Views
Easily Order Medicine Online With Low Price Texas USA
- 2 months ago
- West Virginia, USA
- 38 Views
Buy Online Abortion Pill Pack – Save Up to 50% Off in The UK!
- 3 months ago
- UK
- 25 Views
Exclusive Offer – Order MTP KIT Online – Get 35% Off Discount!
- 3 months ago
- UK
- 20 Views
PediaZone – Happy Tummies with Ranitidine Syrup
- 3 months ago
- Panchkula City, Haryana, India
- 20 Views
Mouth Ulcer Capsule | ICPA Health Products Ltd.
- 4 months ago
- Mumbai - Andheri, Maharashtra, India
- 37 Views
Online Medicine in Hyderabad | Medbuzz
- 4 months ago
- Hyderabad - Secunderabad, Telangana, India
- 42 Views
Buy Gas Tablet Online For Rapid Relief | Suprameds
- 4 months ago
- Hyderabad - Sanathnagar, Telangana, India
- 32 Views
Feel Better Fast with PediaZone’s Drotaverine Syrup
- 4 months ago
- Panchkula City, Haryana, India
- 34 Views
Easy Breathing, Better Living – One Air International’s Dry Powder Inhalers
- 4 months ago
- Panchkula City, Haryana, India
- 60 Views
PediaZone’s Esomeprazole Sachet – Relief For Kids’ Acid Reflux
- 4 months ago
- Panchkula City, Haryana, India
- 125 Views
Best Medicine For Diabetes Online in India | Suprameds
- 5 months ago
- Hyderabad - Sanathnagar, Telangana, India
- 76 Views
Discover Relief with PediaZone’s Bilastine Montelukast Syrup For Children
- 5 months ago
- Panchkula City, Haryana, India
- 62 Views
Pediatric Ranitidine Syrup For Child’s Well-Being | PediaZone
- 6 months ago
- Panchkula City, Haryana, India
- 126 Views
One Air International’s Dry Powder Inhalers – A Breath of Fresh Air
- 6 months ago
- Panchkula City, Haryana, India
- 58 Views
Exporter of Nutraceuticals | Secure Life Pharmaceuticals
- 6 months ago
- Modinagar, Uttar Pradesh, India
- 61 Views
Meropenem Manufacturer and Suppliers in India | JM Laboratories
- 7 months ago
- Solan City, Himachal Pradesh, India
- 62 Views
Fastest Home Delivery Medicines in Bhubaneswar | Ashoka Medicines
- 7 months ago
- Bhubaneswar (M.Corp.), Odisha, India
- 53 Views
Progesterone Soft Gelatin Capsules | Indo Rama Pharma
- 7 months ago
- Solan City, Himachal Pradesh, India
- 72 Views
Best Softgel Capsule of Lycopene | Opsons Biotech
- 8 months ago
- Ambala City, Haryana, India
- 86 Views
Buy Medicines Online in Bhubaneswar | Ashoka Medicines
- 8 months ago
- Bhubaneswar (M.Corp.), Odisha, India
- 72 Views
Best Quality of Softgel Capsule | Opsons Biotech
- 9 months ago
- Ambala City, Haryana, India
- 64 Views
Best Clarithromycin Injection Manufacturers in India | Scotmed Care
- 9 months ago
- Ambala City, Haryana, India
- 55 Views
Buy Votrient 200mg Medicine – Anti Cancer Medicine | Gandhi Medicos
- 10 months ago
- Rajendra Nagar, Delhi, India
- 46 Views
Abirakast Tablet – Abiraterone Acetate 250mg Tablets | Gandhi Medicos
- 10 months ago
- Rajendra Nagar, Delhi, India
- 53 Views
Anti Cancer Medicine Gefitinib Uses, Side Effects, Price | Gandhi Medicos
- 10 months ago
- Rajendra Nagar, Delhi, India
- 58 Views
Vitamin B12 Ampoules For Injection | CompleteOnlinePharmacy.com
- 10 months ago
- Washington, USA
- 79 Views
Glimepiride and Mmetformin Hydrochloride Tablets | Aplonis Healthcare
- 10 months ago
- Chandigarh City, Chandigarh, India
- 59 Views
Glutathione Injections at Home in Hyderabad | Health at Homes
- 10 months ago
- Hyderabad - Punjagutta, Telangana, India
- 59 Views
Pharmaceutical Export Company in India | Primus Pharmaceuticals
- 11 months ago
- Ambala City, Haryana, India
- 51 Views
Hormones and Infertility Third Party Manufacturing | Amagen india
- 11 months ago
- Jaipur City, Rajasthan, India
- 58 Views
Online Palbociclib Purchase: Is it Safe and Legal?
- 11 months ago
- Connecticut, USA
- 71 Views
Buy Abirapro Tablet – Anti Cancer Medicine | Gandhi Medicos
- 11 months ago
- Rajendra Nagar, Delhi, India
- 72 Views
What is Active Pharmaceuticals Ingredients? GN Pal Speciality Molecules LLP
- 11 months ago
- Haldwani, Uttarakhand, India
- 72 Views
PCD Pharma Companies in Telangana | Coniak Lifesciences
- 11 months ago
- Panchkula City, Haryana, India
- 59 Views
Amoxiclav Tablet Manufacturer in India | Medifame Biotech
- 11 months ago
- Panchkula City, Haryana, India
- 71 Views
Leading Private Label Supplement & Vitamins Manufacturer | Natural Private Label
- 11 months ago
- UK
- 56 Views
The Benefits and Risks of Exemestane Purchase: What You Need to Know
- 12 months ago
- Connecticut, USA
- 104 Views
Is Alendronate Right For You? A Purchase Guide
- 12 months ago
- Connecticut, USA
- 68 Views
Leading Pharma Company in Mumbai | Ciron Pharma
- 12 months ago
- Mumbai - Andheri, Maharashtra, India
- 139 Views
Hot Sale 100iu Skin Anti Aging Botox Aller Gan Botox Botu Linum Botax
- 12 months ago
- New Jersey, USA
- 68 Views
Buy Botox Online | Botox Deals Near Me | Bioderglow.com
- 12 months ago
- Connecticut, USA
- 59 Views
Pharmaceutical Company in Chandigarh | Pramiscure Pharmaceuticals
- 1 year ago
- Chandigarh City, Chandigarh, India
- 95 Views
Bacillus Coagulans SNZ 1969 probiotic strains | Sanzyme Biologics
- 1 year ago
- Hyderabad - Banjara Hills, Telangana, India
- 77 Views
Jointo King Capsule For All Kinds of Joint Pains
- 1 year ago
- Sonipat City, Haryana, India
- 161 Views
Sheopals Natural Diabetes Capsule – Control Blood Sugar
- 1 year ago
- Agra City, Uttar Pradesh, India
- 345 Views
3rd Party Pharma Manufacturing
- 1 year ago
- Sas Nagar (Mohali), Punjab, India
- 129 Views
Cipzer Arq Dasharam for Weight Loss & Weakness
- 1 year ago
- Sonipat City, Haryana, India
- 119 Views
Buy IMPCOPS (Siddha-Ayurvedic-Unani) Medicine
- 1 year ago
- Kancheepuram City, Tamil Nadu, India
- 99 Views
Buy Ayurvedic Oil For Joint & Body Pain Relief
- 1 year ago
- Ahmadabad City, Gujarat, India
- 110 Views
Buy Cipzer Jawarish-E-Zanjabeel in India
- 1 year ago
- Sonipat City, Haryana, India
- 91 Views
Majoon-E-Hajr-UI-Yahood For Kidney Stone Remove
- 1 year ago
- Andrews Ganj, Delhi, India
- 129 Views
Buy Re Cell Heal Capsule For Burns and Pains | KBK Herbals
- 2 years ago
- Hyderabad - Uppal, Telangana, India
- 113 Views
Buy Ortho Yes Capsule For Pain Relief | Goyal Ayurveda & Herbals Bhopal
- 2 years ago
- Bhopal City, Madhya Pradesh, India
- 180 Views
India’s First Ayurvedic Supplement For PCOS/PCOD Problem | Solvve
- 2 years ago
- Coimbatore South, Tamil Nadu
- 144 Views
Pharmaceutical Herbal and Ayurvedic Company in India | Zivi Herbals
- 2 years ago
- Panchkula City, Haryana
- 158 Views
Health Veda Organics Plant Based Garcinia Cambogia Supplements
- 2 years ago
- Indore City, Madhya Pradesh
- 142 Views
Health Veda Organics Glucosamine Chondroitin Tablets MSM For Healthy Bones & Joints
- 2 years ago
- Indore City, Madhya Pradesh
- 118 Views
Health Veda Organics Skin Radiance Collagen Builder Capsules For Glowing, Anti-Aging Skin
- 2 years ago
- Indore City, Madhya Pradesh
- 135 Views
Health Veda Organics Ashwagandha Tablets For Anxiety, Stress Relief & Immunity Booster
- 2 years ago
- Indore City, Madhya Pradesh
- 152 Views
Buy Health Veda Organics Apple Cider Vinegar Capsule | HealthVedaOrganics.com
- 2 years ago
- Indore City, Madhya Pradesh
- 146 Views
Buy Lekhniya Tablet For Weight Loss Treatment & Reducing Cholesterol Level
- 2 years ago
- Una City, Himachal Pradesh
- 149 Views
AROGYAM PURE HERBS WEIGHT LOSS KIT
- 2 years ago
- Una City, Himachal Pradesh
- 128 Views
Antibiotics Manufacturer in India | Florencia Healthcare
- 2 years ago
- Noida City, Uttar Pradesh
- 145 Views
Dexlansoprazole DDR Pellets Manufacturers in India | Spansules Pharma
- 2 years ago
- Hyderabad - Secunderabad, Telangana
- 210 Views
Health Veda Organics Diabetic Care Supplements | Manages Blood Sugar
- 2 years ago
- Indore City, Madhya Pradesh
- 226 Views
Health Veda Organics Plant Based Garcinia Cambogia
- 2 years ago
- Indore City, Madhya Pradesh
- 161 Views
Health Veda Organics Glucosamine, Chondroitin, MSM | For Healthy Bones & Joints
- 2 years ago
- Indore City, Madhya Pradesh
- 138 Views
Health Veda Organics Plant Based B Complex | Stress Reliever, Boost Immunity
- 2 years ago
- Indore City, Madhya Pradesh
- 196 Views
Health Veda Organics Apple Cider Vinegar Capsule | Weight Management & Gut Health
- 2 years ago
- Indore City, Madhya Pradesh
- 101 Views
Health Veda Organics Ashwagandha Tablets For Anxiety, Stress Relief & Immunity Booster
- 2 years ago
- Indore City, Madhya Pradesh
- 160 Views
Health Veda Organics Apple Cider Vinegar Capsule – Weight Management & Gut Health
- 2 years ago
- Indore City, Madhya Pradesh
- 137 Views
Health Veda Organics Plant Based B Complex – Stress Reliever, Boost Immunity
- 2 years ago
- Indore City, Madhya Pradesh
- 126 Views
Health Veda Organics Plant Based Garcinia Cambogia
- 2 years ago
- Indore City, Madhya Pradesh
- 137 Views
Health Veda Organics Glucosamine, Chondroitin, MSM For Healthy Bones & Joints
- 2 years ago
- Indore City, Madhya Pradesh
- 140 Views
Expert-Recommended Supplements to Boost Immune System | Ensure india
- 2 years ago
- Bhandara City, Maharashtra
- 194 Views
AROGYAM PURE HERBS WEIGHT LOSS KIT
- 2 years ago
- Una City, Himachal Pradesh
- 204 Views
AROGYAM PURE HERBS KIT FOR PCOS/PCOD
- 2 years ago
- Una City, Himachal Pradesh
- 117 Views
AROGYAM PURE HERBS ALLERGY CURE KIT
- 2 years ago
- Una City, Himachal Pradesh
- 125 Views
Twasa Noni With Aloevera Bath Soap 100gm
- 2 years ago
- Ahmadabad City, Gujarat
- 186 Views
Apollo Noni With Aloe Vera Bath Soap 125gm
- 2 years ago
- Ahmadabad City, Gujarat
- 161 Views
Apollo Noni Toothpaste – Keep Your Teeth & Gums Healthy
- 2 years ago
- Ahmadabad City, Gujarat
- 374 Views
Geo Panch Tulsi Drops – It Helps Boost Immunity
- 2 years ago
- Ahmadabad City, Gujarat
- 302 Views
ApolloNoni Juice – Perfect Family Health Drink
- 2 years ago
- Ahmadabad City, Gujarat
- 318 Views
Zalim Lotion For Ringworm and Itches
- 2 years ago
- Indore City, Madhya Pradesh
- 600 Views
Jeeni Millet Health Mix – Hegde Medical, Dharwad
- 2 years ago
- Dharwad City, Karnataka
- 339 Views
SastaSundar.Com is Now Flipkart Health+
- 2 years ago
- Golmuri-Cum-Jugsalai, Jharkhand
- 390 Views
KAVACHPRASH – Powerful Immunity Booster
- 2 years ago
- Patan City, Gujarat
- 142 Views
Cipla Xgain Anti-Dandruff Shampoo Price on Cureka
- 2 years ago
- Melur, Tamil Nadu
- 255 Views
Glucofort Medicines Support Blood Sugar Levels
- 2 years ago
- Kanyakumari City, Tamil Nadu, India
- 178 Views
Become Fatty Due to Lockdown, Weight Loss – Meticore
- 2 years ago
- Kolhapur City, Maharashtra
- 237 Views
Herbal Health Products By Sukhayu Herbal Pharmacy
- 2 years ago
- Hyderabad - Puppalguda, Telangana
- 209 Views
Herbal Health Products For Natural Healing Of Every Illness
- 2 years ago
- Hyderabad - Puppalguda, Telangana
- 250 Views
AROGYAM PURE HERBS ALLERGY CURE KIT
- 2 years ago
- Una City, Himachal Pradesh
- 228 Views














































































































































You must be logged in to post a review.